App Features
We’ve designed the 1xBet APK to provide everything you need for a complete betting and gaming experience. With 72 sports, 158 casino providers, and support for multiple languages, our app ensures that every player in Bangladesh has access to the best tools and features.
| 1xBet APK | Details |
| App Category | Sports Betting, Online Casino |
| Supported OS | Android, iOS |
| App Version | v.137 (17619) |
| Download Link | 1xbet.apk |
| Betting Options | 72 sports |
| Live Streaming | Directly in the app |
| Casino Options | 158 top providers |
| Welcome Bonus | 120% bonus on your first deposit |
| Promo Code BONUS773 | An extra 30% on your deposit limit |
1xBet APK for Android
The 1xBet app download for Android is optimized for smooth performance on all modern Android devices. Our app provides stable access to sports betting, live events, and casino games, ensuring a seamless experience for every user. With its user-friendly design, it’s easy to enjoy all features right from your phone.
Steps to Download and Install APK
To download 1xBet APK for Android, simply follow these steps for a quick and hassle-free installation, bringing you closer to the ultimate betting experience.

- Visit our website: Open the official 1xBet site and click the mobile icon.
- Select the APK: Choose 1xBet download APK for Android.
- Enable permissions: Allow downloads from unknown sources in your phone settings if prompted.
- Download the file: Click the link and wait for the download to complete.
- Install the app: Open the APK file and follow the steps to finish installation.
Once installed, our app is ready to go. Log in or create an account to access live games, betting options, and exclusive bonuses right away.
Android System Requirements
Our app runs smoothly on most Android devices. Here’s what your phone needs to download 1xBet app:
| Features | Details |
| Operating System | Android 5.0 or higher |
| RAM | 2 GB or more |
| Storage Space | 90 MB free |
| Processor | 1.4 GHz or faster |
Supported Android Devices
The 1xBet APK is optimized to work on a wide range of Android smartphones and tablets. Below is the list of supported devices:
| Smartphones | Tablets |
| Samsung Galaxy S, A, M series | Samsung Galaxy Tab series |
| Google Pixel 3 and newer | Google Nexus series |
| Huawei P, Mate, Honor series | Huawei MediaPad series |
| Xiaomi Redmi, Mi, Poco series | Xiaomi Pad series |
| Oppo Reno, A series | Oppo Pad series |
| Vivo V, Y series | Vivo Pad series |
| OnePlus 5 and newer | Lenovo Tab series |
1xBet App for iOS
The 1xBet app for iOS gives you quick access to betting and gaming. We’ve ensured it’s fully optimized, so you can enjoy sports betting, live events, and casino games with no interruptions. Our app works perfectly on iPhones and iPads, providing everything you need just a tap away.
Steps to Download and Install App
The 1xBet download app process is simple. Follow these steps:

- Visit our website: Open the official 1xBet site and tap on the mobile icon.
- Select iOS version: Choose the 1xBet app download for iOS.
- Download the app: Tap the link and save the file to your device.
- Install the app: Follow the on-screen instructions to complete the installation.
- Log in or sign up: Access your account or create a new one to start playing.
Once installed, our app gives you access to all features, including live betting and exclusive promotions.
iOS System Requirements
We’ve kept the app lightweight so it works on most devices. Here’s what you’ll need for the 1xBet app download in Bangladesh:
| Features | Details |
| Operating System | iOS 8.0 or later |
| RAM | 1 GB or more |
| Storage Space | At least 90 MB free |
| Processor | 1.2 GHz or faster |
Supported iOS Devices
The 1xBet mobile app is compatible with a wide range of Apple devices. Here’s the list:
| iPhone | iPad |
| iPhone 5, 5C, 5S | iPad 2, 3, 4 |
| iPhone 6, 6S, 6 Plus | iPad Air, Air 2 |
| iPhone 7, 7 Plus | iPad Mini 2, 3, 4 |
| iPhone 8, 8 Plus | iPad Pro |
| iPhone X, XR, XS | iPad (5th and 6th Gen) |
Screenshots
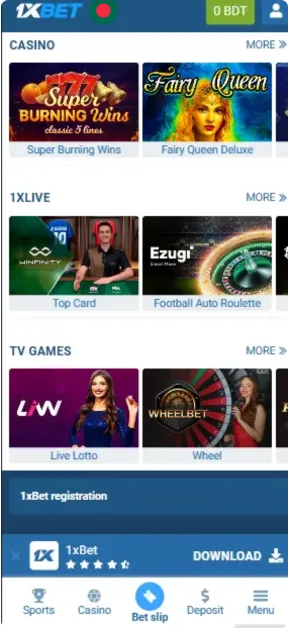
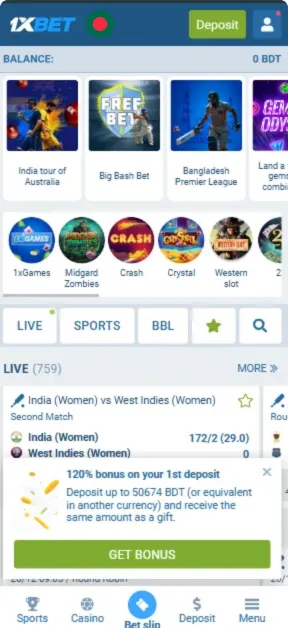
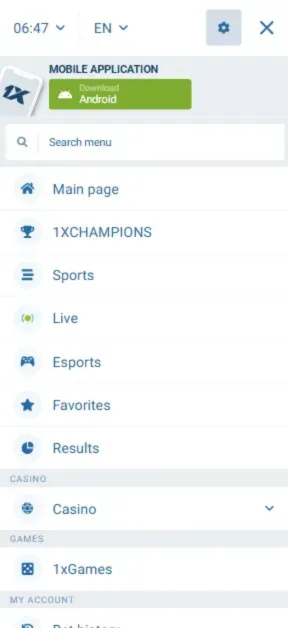
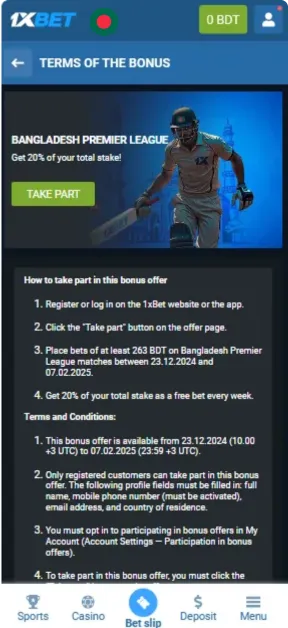

How to Register in the App

To start using the 1xBet app APK, choose one of the four registration methods and fill in the required fields.
- One-click registration: Quick and easy, with minimal details needed.
- Phone number: Register using your mobile number for added security.
- Email: Create an account by entering your email address and setting a password.
- Social networks: Sign up through your preferred social media account.
After completing the registration, you’ll be able to access all features of our app, including betting, casino games, and live streaming.
Make sure to verify your account to withdraw winnings without any delays.
App Functionality
In the app, we offer the full functionality of our website, including sports betting, live events, casino games, financial transactions, and access to bonuses. Everything you need is available in one place, optimized for convenience and ease of use.
Sports Betting
We bring you the ultimate sports betting experience with 72 types of sports and a dedicated section for e-sports. Our app lets you place bets on your favorite events, including Live Betting, so you never miss a moment of action:

- Popular Sports: Football, Basketball, Tennis, Cricket
- Less Common Sports: Darts, Cycling, Table Tennis
- E-sports: Dota 2, CS:GO, League of Legends
- Live Betting: Real-time odds and match updates
- Bet Types: Singles, Accumulators, System Bets
With our app, betting on sports has never been easier. Our users enjoy quick navigation, real-time updates, and competitive odds across all categories.
Casino
Our casino offers everything from classic slots to games with live dealers. With 158 top providers, including Aviator, Zeppelin, and JetX, we give you access to the most exciting titles and real-time gaming experiences.

- Popular Games: Aviator, Zeppelin, JetX
- Slot Providers: NetEnt, Microgaming, Pragmatic Play
- Live Casino: Blackjack, Roulette, Baccarat
- Special Features: Free Spins, Jackpots, Bonus Rounds
- Mobile Optimized: Play seamlessly on any Android or iOS device
Our users love the variety and quality of games we provide. From thrilling slots to real-time games with live dealers, there’s always something exciting waiting for you.
Bonuses for App Users
We offer a variety of exciting bonuses for our users in the app, making every game and bet more rewarding. Check out the top promotions below:

- 120% Bonus on First Deposit: Get up to 50,896 BDT when you make your first deposit.
- Welcome Package: Receive up to 215,012 BDT + 150 Free Spins across four deposits:
- Cricket Free Bet: Place a single bet on the “First Ball Result” market in cricket matches and get a free bet up to 3,937 BDT if your bet loses.
- Lucky 200%: Place bets in 1xGames, and every day, 10,000 lucky bets have their winnings doubled (x2).
Make sure your account is fully registered, with all required fields completed, and confirm your participation in bonus promotions in the account settings.
Deposit and Withdrawal Options
We provide convenient deposit options for our users, ensuring fast and secure transactions. Below is a list of available deposit methods along with their limits:
| Deposit Method | Min-Max Amount (BDT) |
| Bkash | 300.00 – 20,000.00 |
| Nagad | 300.00 – 20,000.00 |
| Upay | 200.00 – 20,000.00 |
| Cellfin | 500.00 – 25,000.00 |
| OK Wallet | 500.00 – 20,000.00 |
| NexusPay | 1,000.00 – 25,000.00 |
| Neteller | 545.19 – 68,148,071.46 |
| Bitcoin | 10 µBTC (approx. 119.59 BDT) |
| Tether | 15 USDT (approx. 1,927.30 BDT) |
For withdrawals, we also offer reliable options with straightforward limits. Below is the list of withdrawal methods and their limits:
| Withdrawal Method | Min-Max Amount (BDT) |
| Bkash | 500.00 – 20,000.00 |
| Nagad | 500.00 – 20,000.00 |
| Upay | 800.00 – 20,000.00 |
| Cellfin | 500.00 – 25,000.00 |
| NexusPay | 2,000.00 – 25,000.00 |
| OK Wallet | 500.00 – 20,000.00 |
| Piastrix | 131.11 – 1,311,086.40 |
We strive to make both deposits and withdrawals simple and efficient, ensuring a smooth experience for all users. Use the 1xBet app for fast transactions and enjoy seamless gameplay.
1xBet App Pros & Cons
The 1xBet app is designed to provide a top-notch betting and gaming experience, but like any app, it has its strengths and areas for improvement. Here’s a quick look:
| Pros | Cons |
| Supports 72 sports and e-sports betting | Requires sufficient storage space (90 MB) |
| Access to 158 casino providers and live dealer games | Not available on Google Play due to restrictions |
| Seamless deposits and withdrawals with local methods | May require updates for compatibility with older devices |
| Live streaming and real-time betting | Internet stability required for live features |
| User-friendly interface with multiple languages, including Bengali | Some advanced features may require a learning curve |
Ready to elevate your betting experience? 1xBet app APK download today and enjoy unmatched convenience, exciting features, and exclusive bonuses—all in one app!
